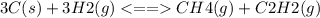Chemistry, 23.07.2020 06:01 quintencoffman2
Consider the following system, which is at equilibrium, 3C(s) + 3H2(g) <--> CH4(g) + C2H2(g) The result of removing some C(s) from the system will be:

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 12:00
What is the percentage of hydrogen in nitrogen trihydride
Answers: 1

Chemistry, 22.06.2019 13:10
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 19:10
Which statement correctly describes the phosphate ion, ? it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms covalently bonded together, and there is a –3 charge on the phosphorus atom. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge distributed over the entire ion. it is composed of one phosphorus atom and four oxygen atoms ionically bonded together, and there is a –3 charge on the phosphorus atom.
Answers: 3
You know the right answer?
Consider the following system, which is at equilibrium, 3C(s) + 3H2(g) <--> CH4(g) + C2H2(g) T...
Questions

Mathematics, 20.07.2019 10:40

Mathematics, 20.07.2019 10:40

Mathematics, 20.07.2019 10:40

Mathematics, 20.07.2019 10:40

Geography, 20.07.2019 10:40

History, 20.07.2019 10:40


Chemistry, 20.07.2019 10:40

English, 20.07.2019 10:40


Biology, 20.07.2019 10:40

History, 20.07.2019 10:40

History, 20.07.2019 10:40


Mathematics, 20.07.2019 10:40

Biology, 20.07.2019 10:40


Mathematics, 20.07.2019 10:40

Mathematics, 20.07.2019 10:40