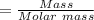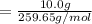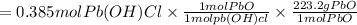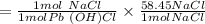Chemistry, 24.07.2020 17:01 tessalopezgarcia2345
Pb(OH)Cl, one of the lead compounds used in ancient Egyptian cosmetics, was prepared from PbO according to the following recipe: PbO(s) NaCl(aq) H2O(l) --> Pb(OH)Cl(s) NaOH(aq) How many grams of PbO and how many grams of NaCl would be required to produce 10.0 g of Pb(OH)Cl

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1


You know the right answer?
Pb(OH)Cl, one of the lead compounds used in ancient Egyptian cosmetics, was prepared from PbO accord...
Questions


English, 09.12.2020 19:50

Arts, 09.12.2020 19:50



English, 09.12.2020 19:50


Arts, 09.12.2020 19:50


History, 09.12.2020 19:50




Geography, 09.12.2020 19:50

English, 09.12.2020 19:50

Mathematics, 09.12.2020 19:50


Mathematics, 09.12.2020 19:50

Mathematics, 09.12.2020 19:50