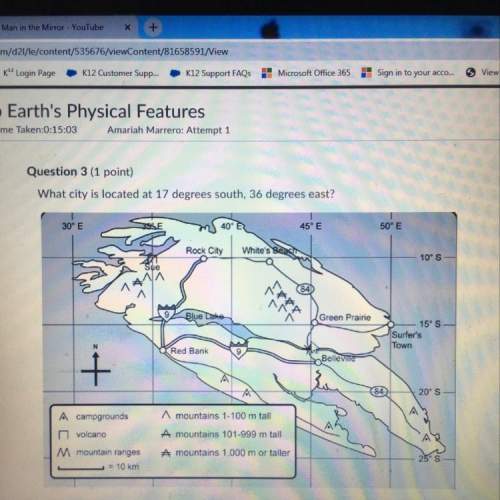
Why is warm honey easier to pour than cold honey?
A) Increasing the temperature breaks the molecules down into atoms, which are smaller.
B) Increasing the temperature increases the kinetic energy of the molecules, which makes it easier to overcome the attractive forces between molecules.
C) Increasing the temperature converts the sugars into water, which flows easier.
D) Increasing the temperature makes the honey more viscous.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
A+b→2c when the reaction begins, the researcher records that the rate of reaction is such that 1 mole of a is consumed per minute. after making changes to the reaction, the researcher notes that 2 moles of a are consumed per minute. what change could the researcher have made to effect this change?
Answers: 1

Chemistry, 22.06.2019 10:50
How many grams of oxygen gas are contained in a 15 l sample at 1.02 atm and 28°c? show your work.
Answers: 1

Chemistry, 22.06.2019 11:10
Which of the following shapes would represent a molecule with two bonded atoms and 3 lone pairs on only one of them , trigonal planar , bent , trigonal pyramidal , linear
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1
You know the right answer?
Why is warm honey easier to pour than cold honey?
A) Increasing the temperature breaks the molecule...
Questions


History, 02.11.2019 00:31

Mathematics, 02.11.2019 00:31

Spanish, 02.11.2019 00:31



Mathematics, 02.11.2019 00:31



Mathematics, 02.11.2019 00:31



Biology, 02.11.2019 00:31


History, 02.11.2019 00:31


Biology, 02.11.2019 00:31

Mathematics, 02.11.2019 01:31

Mathematics, 02.11.2019 01:31