

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
When determining the shape of a molecule, it is important to draw a lewis dot structure first in order to see the total number a. electrons within the moleculeb. bonding and unshared pairs around central atomc. unshared pair within the molecule( i really need it )
Answers: 1

Chemistry, 22.06.2019 00:20
What are the spectator ions in 2h+ + so42- + ca2+ + 2r → caso4 + 2h+ + 21?
Answers: 1

Chemistry, 22.06.2019 03:30
The atomic radius of sodium is 186 pm and of chlorine is 100 pm. the ionic radius for na+ is 102 pm and for cl– is 181 pm. in going from na to cl in period 3, why does the atomic radius decrease while the ionic radius increases? a. the inner electrons in the sodium cation shield its valence electrons more effectively than the inner electrons in the chloride anion do. b. the inner electrons shield the valence electrons more effectively in the chlorine atom than in the chloride anion. c. the outermost electrons in chloride experience a smaller effective nuclear charge than those in the sodium cation do. d. the outermost electrons in chloride experience a larger effective nuclear charge than those in the sodium cation do. e. monatomic ions are bigger than the atoms from which they are formed.
Answers: 2

Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3
You know the right answer?
The following reaction is part of the electron transport chain. Complete the reaction and identify w...
Questions






History, 26.07.2021 05:50

History, 26.07.2021 05:50

Mathematics, 26.07.2021 05:50

Mathematics, 26.07.2021 05:50

Physics, 26.07.2021 05:50


History, 26.07.2021 05:50


Mathematics, 26.07.2021 05:50

Mathematics, 26.07.2021 05:50




Computers and Technology, 26.07.2021 05:50

Mathematics, 26.07.2021 05:50


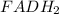
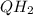 , hence the reducing agent.
, hence the reducing agent. 

