
Chemistry, 25.07.2020 03:01 jessica112776
Use the balanced combustion reaction above to calculate the enthalpy of combustion for C8H16. C8H16(1)= -174.5kJ/mol. I have no clue how to start this question and need help including the formulas so I know how to do it and some step by step commentary.



Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:30
You have a sample of a gas that occupies a volume of 17ml at -111 degrees celsius. what volume does the sample occupy at 88 degrees celsius? show all work asap
Answers: 3

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3

Chemistry, 22.06.2019 09:30
In 2002, the rare earth elements mine in mountain pass, california was closed because
Answers: 1

Chemistry, 22.06.2019 12:30
Suppose you wanted to make 100 grams of water. what is the molar mass of water (h2o)?
Answers: 2
You know the right answer?
Use the balanced combustion reaction above to calculate the enthalpy of combustion for C8H16. C8H16(...
Questions

Mathematics, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40


Chemistry, 27.04.2021 20:40

History, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40



Biology, 27.04.2021 20:40


Mathematics, 27.04.2021 20:40




History, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40

Arts, 27.04.2021 20:40

Mathematics, 27.04.2021 20:40

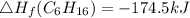
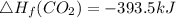
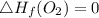
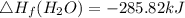
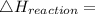 8 x - 393.5 - 8 x 285.82 + 174.5x 1
8 x - 393.5 - 8 x 285.82 + 174.5x 1 

