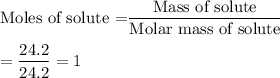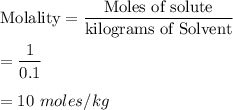Chemistry, 27.07.2020 01:01 meCreation
If you combine 24.2 g of a solute that has a molar mass of 24.2 g/mol with 100.0 g of a solvent, what is the molality of the resulting solution

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 22.06.2019 21:30
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2

Chemistry, 22.06.2019 22:30
Molecular iodine, i2(g), dissociates into iodine atoms at 625 k with a first-order rate constant of 0.271 s−1. part a part complete what is the half-life for this reaction?
Answers: 3

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
If you combine 24.2 g of a solute that has a molar mass of 24.2 g/mol with 100.0 g of a solvent, wha...
Questions




Health, 05.01.2021 07:20

Mathematics, 05.01.2021 07:20

Mathematics, 05.01.2021 07:20

Mathematics, 05.01.2021 07:20


Mathematics, 05.01.2021 07:20

French, 05.01.2021 07:20

English, 05.01.2021 07:20

Mathematics, 05.01.2021 07:20

English, 05.01.2021 07:20

Mathematics, 05.01.2021 07:20


Advanced Placement (AP), 05.01.2021 07:20


Business, 05.01.2021 07:20

Health, 05.01.2021 07:20

Mathematics, 05.01.2021 07:20