
Chemistry, 27.07.2020 01:01 ayoismeisjuam
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazoic acid is 2.20×10−5. Use the method of successive approximations in your calculations or the quadratic formula.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
What type of energy do chemical bonds have? what type of energy is it converted to during chemical reactions? question 15 options: chemical bonds have kinetic energy, which is converted to potential energy during chemical reactions. chemical bonds have electric energy, which is converted to potential energy during chemical reactions. chemical bonds have heat energy, which is converted to kinetic energy during chemical reactions. chemical bonds have potential energy, which is converted to heat energy during chemical reactions.
Answers: 1

Chemistry, 22.06.2019 12:00
What term is applied to a scientist who studies ancient life, including animal and plant fossils a. anthropologist b. dendroclimatologist c. geophysicist d. paleontologist
Answers: 2

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Calculate the [H+] and pH of a 0.0040 M hydrazoic acid solution. Keep in mind that the Ka of hydrazo...
Questions


Mathematics, 11.05.2021 07:10

Chemistry, 11.05.2021 07:10



History, 11.05.2021 07:10

Chemistry, 11.05.2021 07:10







Biology, 11.05.2021 07:10




Mathematics, 11.05.2021 07:10

Mathematics, 11.05.2021 07:10

Computers and Technology, 11.05.2021 07:10

![[H^+]=0.000285](/tpl/images/0713/5320/5d625.png)
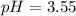
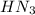 ). So:
). So:
![Ka=\frac{[H^+][N_3^-]}{[HN_3]}](/tpl/images/0713/5320/574a9.png)
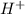 produced we will have 1 mol of
produced we will have 1 mol of 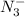 . So, we can use "X" for the unknown values and replace in the Ka equation:
. So, we can use "X" for the unknown values and replace in the Ka equation:![Ka=\frac{X*X}{[HN_3]}](/tpl/images/0713/5320/c6a9a.png)
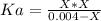
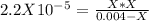
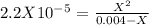
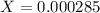
![pH=-Log[H^+]=-Log[0.000285]=3.55](/tpl/images/0713/5320/8cdec.png)


