

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:00
Agas in a balloon at constant pressure has a volume of 160 ml at -125*c. what is its volume at 29.0*c?
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

You know the right answer?
The equilibrium constants for the chemical reaction N 2(g) + O 2(g) 2NO(g) are K P = 1.1 × 10 –3 and...
Questions

Spanish, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40

Chemistry, 01.04.2021 20:40

English, 01.04.2021 20:40


Mathematics, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40

History, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40



English, 01.04.2021 20:40


Mathematics, 01.04.2021 20:40

Mathematics, 01.04.2021 20:40




Computers and Technology, 01.04.2021 20:40

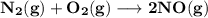
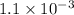
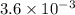
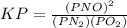
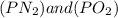 is defined in the denominator section and the value of
is defined in the denominator section and the value of 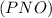 is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).
is defined in the numerator section that defines the value of KP is increases.so, the temperature of the KP will be KP ∝ (PNO).

