
Chemistry, 27.07.2020 01:01 tammydbrooks43
1. What is the net ionic equation for the reaction that occurs when aqueous solutions of AgNO3 and CaCl2 are mixed and a precipitate forms? A. Ca+2(aq) + NO3-(aq) Ca(NO3)2(aq) B. Ag2+(aq) + 2Cl-(aq) AgCl2(s) C. Cl−(aq) + Ag+(aq) ⟶ AgCl(s) D. None of the above because no reaction occurs

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Predict the products of the following acid-base reactions, and predict whether the equilibrium lies to the left or to the right of the reaction arrow.part ao2-(aq)+h2o(l)< => express your answer as part of a chemical equation. identify all of the phases in your answer.o2-(aq)+h2o(l) < => oh-(aq)+oh-(aq)part bpredict whether the equilibrium lies to the left or to the right of the equation in previous part.h2o is a stronger acid than oh–, so the equilibrium lies to the right.h2o is a weaker acid than oh–, so the equilibrium lies to the left.h2o is a stronger acid than oh–, so the equilibrium lies to the left.h2o is a weaker acid than oh–, so the equilibrium lies to the right.part cch3cooh(aq)+hs? (aq) < => express your answer as part of a chemical equation. identify all of the phases in your answer.ch3cooh(aq)+hs-(aq) < => h2s(aq)+c2h3o2-(aq)h2s(aq)+c2h3o2-(aq)part dpredict whether the equilibrium lies to the left or to the right of the equation in previous part.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the right.ch3cooh is a weaker acid than h2s, so the equilibrium lies to the left.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the right.ch3cooh is a stronger acid than h2s, so the equilibrium lies to the left.part eno2-(aq)+h2o(l) < => express your answer as part of a chemical equation. identify all of the phases in your answer.no2-(aq)+h2o(l) < => part fpredict whether the equilibrium lies to the left or to the right of the equation in previous part.hno2 is a stronger acid than h2o, so the equilibrium lies to the right.hno2 is a weaker acid than h2o, so the equilibrium lies to the left.hno2 is a stronger acid than h2o, so the equilibrium lies to the left.hno2 is a weaker acid than h2o, so the equilibrium lies to the right.
Answers: 1


Chemistry, 22.06.2019 16:00
No copying 15 pts how does a free-body diagram tell you about the net force on an object?
Answers: 2

You know the right answer?
1. What is the net ionic equation for the reaction that occurs when aqueous solutions of AgNO3 and C...
Questions




History, 28.09.2019 04:50


Mathematics, 28.09.2019 04:50






Social Studies, 28.09.2019 04:50



Health, 28.09.2019 04:50


History, 28.09.2019 04:50

Advanced Placement (AP), 28.09.2019 04:50

Mathematics, 28.09.2019 04:50

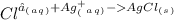
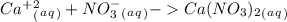
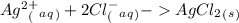
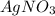 are:
are: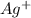 and
and 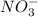
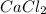 are:
are: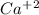 and
and 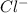
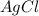 and
and 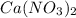
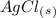 and an aqueous state for
and an aqueous state for 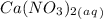 .
. 

