
Chemistry, 27.07.2020 17:01 carafaith02
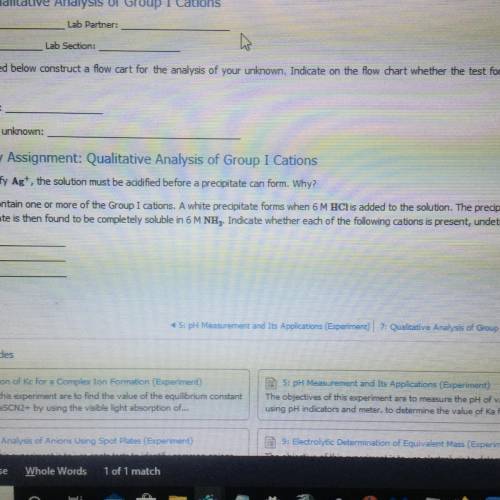
A solution may contain one or more of the Group I cations. A white precipitate forms when 6 M HCl is added to the solution. The precipitate is insoluble in hot
water. The precipitate is then found to be completely soluble in 6M NHIndicate whether each of the following cations is present, undetermined, or absent.
• Pb2+
• Ag+
HG22+
Explain

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which part of earth’s surface receives the most direct rays from the sun? a) equator b) ocean c) poles d) mountains
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

You know the right answer?
A solution may contain one or more of the Group I cations. A white precipitate forms when 6 M HCl is...
Questions


History, 27.09.2019 08:00


Chemistry, 27.09.2019 08:00



History, 27.09.2019 08:00


Social Studies, 27.09.2019 08:10




History, 27.09.2019 08:10

Mathematics, 27.09.2019 08:10

Spanish, 27.09.2019 08:10


Mathematics, 27.09.2019 08:10

Mathematics, 27.09.2019 08:10





