
Chemistry, 28.07.2020 05:01 FavvBella84
For the reaction C + 2H2 - CH2, how many moles of hydrogen are required to produce
19.26 mol of methane, CHA?
Select one:
O a. 19.26
O b. 38.52
O c. 15.0
O d. 24.7

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 20:30
Identify the correct mole ratio for each substance. sodium chloride (nacl) na: cl = 1: ammonium nitrate (nhno) h: o = 4:
Answers: 1


Chemistry, 23.06.2019 07:00
What are the trends and exceptions to the trends in electron affinity?
Answers: 1
You know the right answer?
For the reaction C + 2H2 - CH2, how many moles of hydrogen are required to produce
19.26 mol of met...
Questions

History, 10.11.2019 17:31


Social Studies, 10.11.2019 17:31

Mathematics, 10.11.2019 17:31








Biology, 10.11.2019 17:31

Physics, 10.11.2019 17:31

Mathematics, 10.11.2019 17:31


Social Studies, 10.11.2019 17:31

Biology, 10.11.2019 17:31


Geography, 10.11.2019 17:31

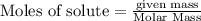
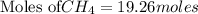
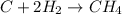
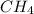 is produced by = 2 moles of
is produced by = 2 moles of 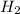
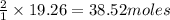 of
of 


