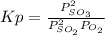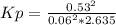Chemistry, 28.07.2020 20:01 arnold2619
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid synthesis. An industrial chemist studying this reaction fills a 1.5 L flask with 0.59 atm of sulfur dioxide gas and 2.9 atm of oxygen gas at 35.0 °C. He then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of sulfur trioxide gas to be 0.53 atm.
Calculate the pressure equilibrium constant for the reaction of sulfur dioxide and oxygen at the final temperature of the mixture. Round your answer to 2 significant digits.
Kp=.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:40
*will mark you brainliest + 15 points ** why does the equilibrium of a system shift when the pressure is increased? a. to maximize the stress on the system b. to stop restoring equilibrium to the system c. to increase the total moles of gas in the system d. to decrease the total moles of gas in the system
Answers: 3

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 20:00
What happens to the temperature of a substance when the average kinetic energy of its particles increases?
Answers: 3

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
Sulfur dioxide and oxygen react to form sulfur trioxide during one of the key steps in sulfuric acid...
Questions

Mathematics, 16.09.2019 12:30





Chemistry, 16.09.2019 12:30



English, 16.09.2019 12:30





Social Studies, 16.09.2019 12:30

Mathematics, 16.09.2019 12:30


English, 16.09.2019 12:30