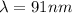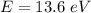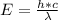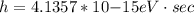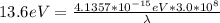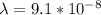Chemistry, 29.07.2020 04:01 solikhalifeoy3j1r
An energy of 13.6 eV is needed to ionize an electron from the ground state of a hydrogen atom. Selecting the longest wavelength that will work from the those given below, what wavelength is needed if a photon accomplishes this task

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Covalent bonds are formed between metals and boiling points true or false
Answers: 2

Chemistry, 22.06.2019 09:30
Mike and mitchell decide to have a foot race. they mark off a stretch of 100 yards, and recruit cindy to work the stopwatch. after running the race and looking at the results, cindy declared that mitchell was the fastest. so how did the boys times compare?
Answers: 3


Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1
You know the right answer?
An energy of 13.6 eV is needed to ionize an electron from the ground state of a hydrogen atom. Selec...
Questions

Mathematics, 30.10.2020 18:00

Mathematics, 30.10.2020 18:00


Geography, 30.10.2020 18:00


Social Studies, 30.10.2020 18:00

History, 30.10.2020 18:00

SAT, 30.10.2020 18:00

Mathematics, 30.10.2020 18:00

Mathematics, 30.10.2020 18:00

Mathematics, 30.10.2020 18:00

Mathematics, 30.10.2020 18:00


History, 30.10.2020 18:00





Advanced Placement (AP), 30.10.2020 18:00