
Chemistry, 29.07.2020 05:01 graycelynn123
A mixture of water and graphite is heated to 600 K in a 1 L container. When the system comes to equilibrium it contains 0.17 mol of H2, 0.17 mol of CO, 0.74 mol of H2O, and some graphite. Some O2 is added to the system and a spark is applied so that the H2 reacts completely with the O2.
Find the amount of CO in the flask when the system returns to equilibrium.
Express your answer to two significant figures and include the appropriate units.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1


Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
A mixture of water and graphite is heated to 600 K in a 1 L container. When the system comes to equi...
Questions



Mathematics, 24.08.2021 15:10

Social Studies, 24.08.2021 15:10

Mathematics, 24.08.2021 15:10


Health, 24.08.2021 15:10


Chemistry, 24.08.2021 15:10

Chemistry, 24.08.2021 15:10


Business, 24.08.2021 15:10



Computers and Technology, 24.08.2021 15:10



Chemistry, 24.08.2021 15:10


Mathematics, 24.08.2021 15:10

![K_c= \dfrac{[CO][H_2]}{[H_2O]}](/tpl/images/0714/6363/51350.png)
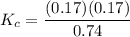
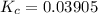
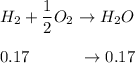
![0.03905 = \dfrac{[0.17+x][x]}{[0.91 -x]}](/tpl/images/0714/6363/fa6d0.png)


