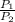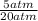Chemistry, 29.07.2020 05:01 miannasanderso8146
2) 2.5 mol of an ideal gas at 20 oC under 20 atm pressure, was expanded up to 5 atm pressure via; (a) adiabatic reversible and (b) adiabatic irreversible process. Calculate the values of w, q, ΔU, ΔH for each process. (Cv = 5 cal / mol. K ≈ 5/2 R; R ≈ 2 cal / mol. K) (Please find the desired values by making the corresponding derivations

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 06:00
In an investigation that uses the scientific method, which step immediately follows making a hypothesis? o summarizing the results o asking a question o making observations designing an experiment mark this and retum save and exit next submit
Answers: 2

Chemistry, 22.06.2019 11:50
The chemical bond connecting one nucleotide with the next one along the nucleic acid chain is called a
Answers: 3

Chemistry, 23.06.2019 03:30
In general metals get as you move from left to right across the periodic table.
Answers: 1
You know the right answer?
2) 2.5 mol of an ideal gas at 20 oC under 20 atm pressure, was expanded up to 5 atm pressure via; (a...
Questions


Mathematics, 13.04.2021 05:50


Arts, 13.04.2021 05:50

Mathematics, 13.04.2021 05:50


Mathematics, 13.04.2021 05:50

Business, 13.04.2021 05:50

Mathematics, 13.04.2021 05:50

Mathematics, 13.04.2021 05:50

Mathematics, 13.04.2021 05:50


Biology, 13.04.2021 05:50

Biology, 13.04.2021 05:50



Chemistry, 13.04.2021 05:50

Mathematics, 13.04.2021 05:50


Mathematics, 13.04.2021 05:50