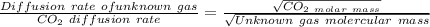Chemistry, 29.07.2020 21:01 Sparkleskeepsgoing
A gas of unknown identity diffuses at a rate of 155 mL/s in a diffusion apparatus in which carbon dioxide diffuses at the rate of 102 mL/s. Calculate the molecular mass of the unknown gas.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1

Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
A gas of unknown identity diffuses at a rate of 155 mL/s in a diffusion apparatus in which carbon di...
Questions

Health, 28.11.2021 23:50




Mathematics, 28.11.2021 23:50


Business, 28.11.2021 23:50

Mathematics, 28.11.2021 23:50




Biology, 28.11.2021 23:50


History, 28.11.2021 23:50





Chemistry, 29.11.2021 01:00