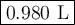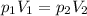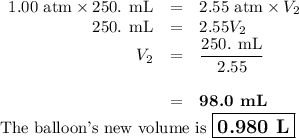Chemistry, 30.07.2020 01:01 Multidewi3540
The gas in a 250. mL piston experiences a change in pressure from 1.00 atm to 2.55 atm. What is the new volume (in mL) assuming the moles of gas and temperature are held constant?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If 1.63 times 10 negative 4 of helium dissolves in 100.0g of water, what is the concentration in parts per million
Answers: 3

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 14:30
Which of the following describes a situation where competition between producers exists
Answers: 1
You know the right answer?
The gas in a 250. mL piston experiences a change in pressure from 1.00 atm to 2.55 atm. What is the...
Questions


Mathematics, 14.11.2020 16:10




Mathematics, 14.11.2020 16:10


History, 14.11.2020 16:10

Biology, 14.11.2020 16:10

Mathematics, 14.11.2020 16:10

Computers and Technology, 14.11.2020 16:10

Mathematics, 14.11.2020 16:10

Chemistry, 14.11.2020 16:10

Chemistry, 14.11.2020 16:10


History, 14.11.2020 16:10

Mathematics, 14.11.2020 16:10



Mathematics, 14.11.2020 16:10