

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Adrop of acetone (nail polish remover) has a mass of 35 mg and a density of 0.788 g/cm3. what is its volume in cubic centimeters?
Answers: 3

Chemistry, 22.06.2019 06:40
Ted and emily played a mixed doubles tennis match against jack and brenda. in the second match. ted and brenda played against jack and emily. which type of chemical reaction does the situation demonstrate?
Answers: 3


Chemistry, 22.06.2019 21:00
One similarity and one difference between an element and a mixture of elements
Answers: 1
You know the right answer?
A student mixes 43.8 mL of acetone (58.08 g/mol, 0.791 g/mL) with excess benzaldehyde and NaOH to pr...
Questions

Computers and Technology, 30.08.2019 06:10

Mathematics, 30.08.2019 06:10


Physics, 30.08.2019 06:10


Mathematics, 30.08.2019 06:10


English, 30.08.2019 06:10

Computers and Technology, 30.08.2019 06:10



Chemistry, 30.08.2019 06:10

Mathematics, 30.08.2019 06:10

Mathematics, 30.08.2019 06:10




Mathematics, 30.08.2019 06:10

Biology, 30.08.2019 06:10

History, 30.08.2019 06:10

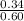 ˣ 100 = 56.67%
ˣ 100 = 56.67%


