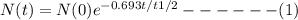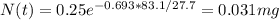Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.036m naoh best answer will be brainliest
Answers: 3

Chemistry, 22.06.2019 23:00
Which type of intermolecular attractions holds ammonia molecules together with other ammonia molecules?
Answers: 3

Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
Chromium-51 is a radioactive isotope used to label red blood cells to identify if they remain intact...
Questions




Mathematics, 04.04.2020 12:49



Mathematics, 04.04.2020 12:50




Mathematics, 04.04.2020 12:50


Mathematics, 04.04.2020 12:50


Mathematics, 04.04.2020 12:51