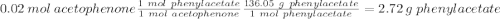Chemistry, 01.08.2020 02:01 bthakkar25
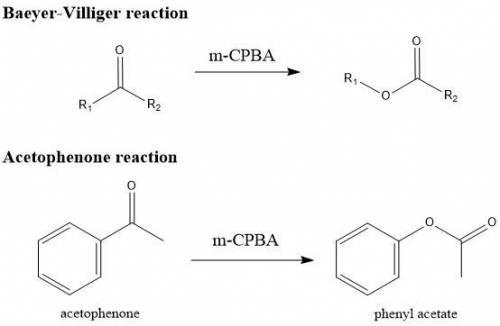
Bayer Villiger Provide a balanced chemical equation of the reaction performed in this experiment. Use structures and compound names to show ALL reactants and products involved. Baeyer-Villiger Reaction of Acetophenone Data Results
• Moles of acetophenone used: (Show calculations) 0.020 moles (2.40g/120.151 g mol-1 =0.0199 moles)
• Moles of mCPBA used: (Show calculations) 0.036 moles_(6.25 grams/ 172.56 g. mol-1)
• Expected mass of the product: (Show calculation. Clearly show the limiting and excess reactants)

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Calculate the h3o+ concentration in a solution of acetic acid if the concentration of molecular acetic acid present at equilibrium is 9.97x10^-3 m and k for the dissociation is 1.86x10^-5. ch3cooh(aq)+h2o(> h3o^+(aq)+ch3coo^-(aq)
Answers: 2


Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1
You know the right answer?
Bayer Villiger Provide a balanced chemical equation of the reaction performed in this experiment. Us...
Questions

Mathematics, 10.02.2021 02:00

Mathematics, 10.02.2021 02:00

Social Studies, 10.02.2021 02:00

Mathematics, 10.02.2021 02:00


History, 10.02.2021 02:00



Health, 10.02.2021 02:00


English, 10.02.2021 02:00

Mathematics, 10.02.2021 02:00

Mathematics, 10.02.2021 02:00

English, 10.02.2021 02:00

History, 10.02.2021 02:00

Mathematics, 10.02.2021 02:00

Business, 10.02.2021 02:00

History, 10.02.2021 02:00

Social Studies, 10.02.2021 02:00

English, 10.02.2021 02:00