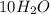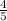Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 04:30
Using the periodic table, complete the table to describe each atom. type in your answers
Answers: 3


Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1
You know the right answer?
n-Butane (C4H10) is burned with stoichiometric amount of oxygen. Determine the mole fraction of carb...
Questions

French, 15.09.2021 19:50

Mathematics, 15.09.2021 19:50

Chemistry, 15.09.2021 19:50

Mathematics, 15.09.2021 19:50

Mathematics, 15.09.2021 19:50

Biology, 15.09.2021 19:50

English, 15.09.2021 19:50


Mathematics, 15.09.2021 19:50


Mathematics, 15.09.2021 19:50

English, 15.09.2021 19:50

History, 15.09.2021 19:50

Mathematics, 15.09.2021 19:50




History, 15.09.2021 19:50

Mathematics, 15.09.2021 19:50

Mathematics, 15.09.2021 19:50

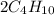 +
+ 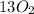 →
→ 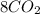 +
+