
Chemistry, 03.08.2020 14:01 zacharoo10
For the following equilibrium, Ag3PO4(s)↽−−⇀3Ag+(aq)+PO3−4(aq) If Ksp=2.4×10−28, what is the molar solubility of Ag3PO4? Report your answer in scientific notation with the correct number of significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
What are the first three quantum numbers for the electrons located in subshell 2s?
Answers: 2

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 23.06.2019 00:00
This statement about matter and its behavior is best classified as a
Answers: 1
You know the right answer?
For the following equilibrium, Ag3PO4(s)↽−−⇀3Ag+(aq)+PO3−4(aq) If Ksp=2.4×10−28, what is the molar s...
Questions


Social Studies, 24.10.2019 19:43


Social Studies, 24.10.2019 19:43




Spanish, 24.10.2019 19:43


Mathematics, 24.10.2019 19:43

Biology, 24.10.2019 19:43



Mathematics, 24.10.2019 19:43



History, 24.10.2019 19:43



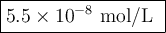
![K_{sp} =\text{[Ag$^{+}$]$^{3}$[PO$_{4}^{3-}$]} = (3x)^{3}x = 2.4 \times 10^{-28}\\27x^{4} = 2.4 \times 10^{-28}\\x^{4} = 8.89 \times 10^{-30}\\x = \sqrt[4]{8.89 \times 10^{-30}}\\= \mathbf{5.5\times 10^{-8}} \textbf{ mol/L}\\\text{The molar solubility of silver phosphate is $\large \boxed{\mathbf{5.5\times 10^{-8}}\textbf{ mol/L }}$}](/tpl/images/0716/8807/790d1.png)


