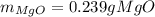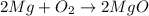Chemistry, 04.08.2020 14:01 denisebaslee15
· A 0.100g sample of Mg when combined with O2 yields 0.166g of Mgo, a
second Mg sample with a mass of 0.144g is also combined with O2. What
mass of MgO is produced from the second sample?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 21:30
In science class richard learns that a substance has a boiling point of 230 fahrenheit his teacher ask him to convert this temperature to degrees celsius what is the boiling point of his substance in degrees celsius
Answers: 3

Chemistry, 23.06.2019 01:20
Use the de broglie's wave equation to find the wavelength of an electron moving at 4.5 × 106 m/s. show your work. note: h= plank's constant (6.62607 x 10-34 j s)
Answers: 1

Chemistry, 23.06.2019 02:00
Now look at the segment of the graph between the two data points marked with black squares. describe how the boiling point and melting point plots behave between these points. be as specific as possible.
Answers: 1
You know the right answer?
· A 0.100g sample of Mg when combined with O2 yields 0.166g of Mgo, a
second Mg sample with a mass...
Questions

English, 04.11.2019 22:31

Mathematics, 04.11.2019 22:31

Business, 04.11.2019 22:31


Mathematics, 04.11.2019 22:31

Chemistry, 04.11.2019 22:31

English, 04.11.2019 22:31



History, 04.11.2019 22:31

History, 04.11.2019 22:31


Mathematics, 04.11.2019 22:31

History, 04.11.2019 22:31

Biology, 04.11.2019 22:31

Physics, 04.11.2019 22:31

Mathematics, 04.11.2019 22:31



Mathematics, 04.11.2019 22:31