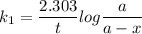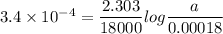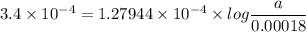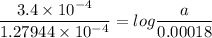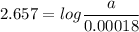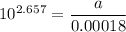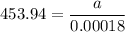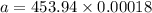Chemistry, 03.08.2020 14:01 lenniestreet10
For a first-order reaction, A → B, the rate coefficient was found to be 3.4 × 10-4 s-1 at 23 °C. After 5.0 h, the concentration of A was found to be 0.00018 mol L-1. What was the original concentration of A?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 23.06.2019 03:30
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

Chemistry, 23.06.2019 03:50
How many liters of oxygen gas, at standardtemperature and pressure, will react with 35.8 grams ofiron metal? 4 fe (s) + 3 o2 (g) → 2 fe2o3 (s)
Answers: 3
You know the right answer?
For a first-order reaction, A → B, the rate coefficient was found to be 3.4 × 10-4 s-1 at 23 °C. Aft...
Questions


Mathematics, 29.04.2021 21:20


Mathematics, 29.04.2021 21:20


Chemistry, 29.04.2021 21:20

Mathematics, 29.04.2021 21:20

Mathematics, 29.04.2021 21:20


Mathematics, 29.04.2021 21:20

Social Studies, 29.04.2021 21:20

Mathematics, 29.04.2021 21:20






Biology, 29.04.2021 21:20

Mathematics, 29.04.2021 21:20

Mathematics, 29.04.2021 21:20