
Chemistry, 04.08.2020 14:01 rajeeblagrove
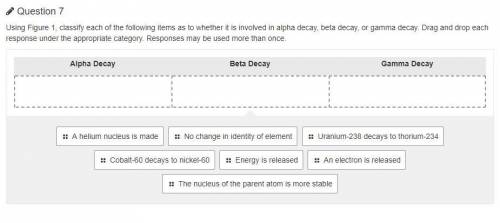
Using Figure 1, classify each of the following items as to whether it is involved in alpha decay, beta decay, or gamma decay. Drag and drop each response under the appropriate category. Responses may be used more than once.


Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:30
Asample contains 16.75 g of the radioisotope u-236 and 50.25 g of its daughter isotope, th-232. how long did it take for decay to take place if one half-life of u-236 is 23 million years? 46 million years 69 million years 92 million years 115 million years
Answers: 3

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3


Chemistry, 23.06.2019 06:30
What is the chemical formula for a compound between li and br? libr li2br libr2 libr3
Answers: 1
You know the right answer?
Using Figure 1, classify each of the following items as to whether it is involved in alpha decay, be...
Questions

Chemistry, 20.10.2019 21:30

Computers and Technology, 20.10.2019 21:30

History, 20.10.2019 21:30

English, 20.10.2019 21:30



Biology, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30



Biology, 20.10.2019 21:30


Mathematics, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30

Mathematics, 20.10.2019 21:30


Mathematics, 20.10.2019 21:30




