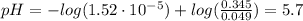Chemistry, 05.08.2020 02:01 montgomerykarloxc24x
A 1.30 L buffer solution consists of 0.107 M butanoic acid and 0.345 M sodium butanoate. Calculate the pH of the solution following the addition of 0.075 moles of NaOH . Assume that any contribution of the NaOH to the volume of the solution is negligible. The Ka of butanoic acid is 1.52×10−5 .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 12:50
Which phrase best describes the rock's texture? 1.jagged grains 2.coarse grains 3.rounded grains 4.non-banded grains
Answers: 1

Chemistry, 21.06.2019 20:30
18. use the activity series to predict whether the following synthesis reaction will occur. write the chemical equations for the reaction if it's predicted to occur. (s) + o2(g) -> *note: it is possible.*
Answers: 1

Chemistry, 21.06.2019 22:00
Which produce would best increase the amount of heat energy that is actually gained by calorimeter b
Answers: 1

You know the right answer?
A 1.30 L buffer solution consists of 0.107 M butanoic acid and 0.345 M sodium butanoate. Calculate t...
Questions


History, 06.02.2021 04:20

Mathematics, 06.02.2021 04:20







Mathematics, 06.02.2021 04:20

Mathematics, 06.02.2021 04:20

Mathematics, 06.02.2021 04:20





Biology, 06.02.2021 04:20

Mathematics, 06.02.2021 04:20


Mathematics, 06.02.2021 04:20

![pH = pKa + log(\frac{[NaC_{4}H_{7}O_{2}]}{[C_{4}H_{8}O_{2}]})](/tpl/images/0717/5530/43ba8.png) (2)
(2)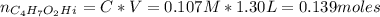
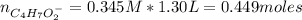
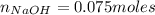
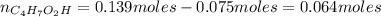
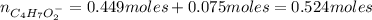
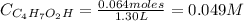
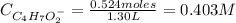
![Ka = \frac{[C_{4}H_{7}O_{2}^{-}][H_{3}O^{+}]}{C_{4}H_{7}O_{2}H}](/tpl/images/0717/5530/cf02c.png)
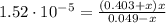
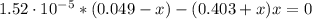
![[C_{4}H_{7}O_{2}^{-}] = 0.345 + 1.85 \cdot 10^{-6} = 0.345 M](/tpl/images/0717/5530/d7dde.png)
![[C_{4}H_{7}O_{2}H] = 0.049 - 1.85 \cdot 10^{-6} = 0.049 M](/tpl/images/0717/5530/eb223.png)