
Chemistry, 12.08.2020 06:01 munziruddin204
Consider the following: Li(s) + ½ I₂(g) --> LiI(s) ΔH = –292 kJ. LiI(s) has a lattice energy of –753 kJ/mol. The ionization energy of Li(g) is 520 kJ/mol, the bond energy of I₂(g) is 151 kJ/mol, and the electron affinity of I(g) is –295 kJ/mol. Use these data to determine the heat of sublimation of Li(s).

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:30
If the root word engage means “to connect with something,” what does the word disengage mean in the following sentence? he disengaged the gears by stepping on the clutch pedal.a.added more engine powerb.activated a connection to the pedalc.stalled the engined.released a connection to the pedal
Answers: 1

Chemistry, 22.06.2019 21:00
Read "who built the pyramids? ”. leave this link open while you answer the questions throughout the assignment. give at least two reasons why some people claim the pyramids of giza were constructed by aliens.
Answers: 1

Chemistry, 23.06.2019 01:00
Substance 33°f 100°f peanut oil solid liquid margarine solid liquid chocolate chips solid liquid which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Consider the following: Li(s) + ½ I₂(g) --> LiI(s) ΔH = –292 kJ. LiI(s) has a lattice energy of –...
Questions





Spanish, 20.10.2020 02:01


Biology, 20.10.2020 02:01


History, 20.10.2020 02:01

Business, 20.10.2020 02:01



History, 20.10.2020 02:01



Mathematics, 20.10.2020 02:01


Mathematics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01

Mathematics, 20.10.2020 02:01

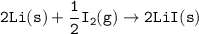
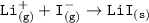
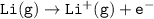
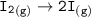
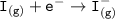
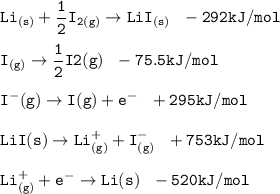
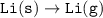 = (-292 +(-75.5)+295+753+(-520)) kJ/mol
= (-292 +(-75.5)+295+753+(-520)) kJ/mol

