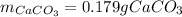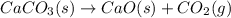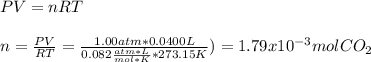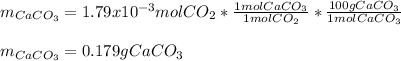Chemistry, 12.08.2020 06:01 DASASDAEDWEDA
Determine the mass of CaCO3 required to produce 40.0 mL CO2 at STP. Hint use molar volume of an ideal gas (22.4 L)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Agas has a volume of 0.7 l at 300 mmhg. what would be the new volume at 900 mmhg
Answers: 1

Chemistry, 22.06.2019 02:30
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 05:00
Cucl2 + 2nano3 cu(no3)2 + 2nacl what is the percent yield of nacl if 31.0 g of cucl2 reacts with excess nano3 to produce 21.2 g of nacl? 49.7% 58.4% 63.6% 78.7%
Answers: 1

Chemistry, 23.06.2019 03:50
How many moles of potassium are needed to react completely with 12.8 moles of magnessium bromide?
Answers: 2
You know the right answer?
Determine the mass of CaCO3 required to produce 40.0 mL CO2 at STP. Hint use molar volume of an idea...
Questions

Computers and Technology, 24.11.2020 23:50

Mathematics, 24.11.2020 23:50


English, 24.11.2020 23:50

Mathematics, 24.11.2020 23:50

Mathematics, 24.11.2020 23:50


Mathematics, 24.11.2020 23:50


Chemistry, 24.11.2020 23:50


Spanish, 24.11.2020 23:50

Mathematics, 24.11.2020 23:50


English, 24.11.2020 23:50

Health, 24.11.2020 23:50


Mathematics, 24.11.2020 23:50