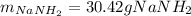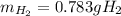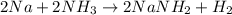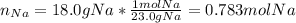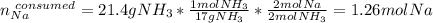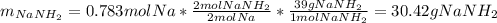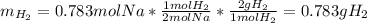Chemistry, 12.08.2020 05:01 mathwiznot45
Assuming that you start with 21.4 g of ammonia gas and 18.0 g of sodium metal and assuming that the reaction goes to completion, determine the mass (in grams) of each product.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:10
Think about how you can use le chatelier’s principle to find possible solutions to the design problem. describe at least two ways to increase the yield (amount) of ammonia based on this principle.
Answers: 2

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 18:30
Two people each hold the end of a rope and create waves by moving their arms up and down. this wave is best classified as a transverse wave because a) both the rope particles and the wave are moving in the same direction. b) the wave is moving up and down as the particles of the rope move horizontally. c) the wave is moving horizontally as the particles of the rope move up and down. eliminate d) the wave is moving in a parallel direction with the motion of the person's arms.
Answers: 3
You know the right answer?
Assuming that you start with 21.4 g of ammonia gas and 18.0 g of sodium metal and assuming that the...
Questions

Mathematics, 26.05.2020 02:02


History, 26.05.2020 02:02

Mathematics, 26.05.2020 02:02

Mathematics, 26.05.2020 02:02


History, 26.05.2020 02:02


History, 26.05.2020 02:02



Biology, 26.05.2020 02:02


Spanish, 26.05.2020 02:02

Mathematics, 26.05.2020 02:02



Mathematics, 26.05.2020 02:02

Mathematics, 26.05.2020 02:02