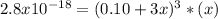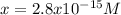Chemistry, 12.08.2020 06:01 shanilafaridor97hl
The solubility product for Ag3PO4 is 2.8 × 10‑18. What is the solubility of silver phosphate in a solution which also contains 0.10 moles of silver nitrate per liter?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:20
Calculate the molarity of 48.0 ml of 6.00 m h2so4 diluted to 0.250 l .
Answers: 1

Chemistry, 22.06.2019 05:00
1)each group 16 element has how many valence electrons? ( )4 ( )6 ( )8 ( )16 2)how many dots appear in the dot structure for calcium ion, ca2+? ( )zero ( )one ( )two ( )eight 3) which of the following atoms forms a cation to obtain an octet of outer shell electrons? ( )magnesium ( )oxygen ( )fluorine ( )helium 4) an al3+ ion contains 13 protons and 10 electrons. ( )true ( )false 5) valence and non-valence electrons are represented in lewis dot structures. ( )true ( )false
Answers: 3


Chemistry, 22.06.2019 17:00
The atoms of a solid aluminum can are close together, vibrating in a rigid structure. if the can is warmed up on a hot plate, what happens to the atoms?
Answers: 1
You know the right answer?
The solubility product for Ag3PO4 is 2.8 × 10‑18. What is the solubility of silver phosphate in a so...
Questions

Mathematics, 21.04.2021 22:00

History, 21.04.2021 22:00


Mathematics, 21.04.2021 22:00


Mathematics, 21.04.2021 22:00

Health, 21.04.2021 22:00

Mathematics, 21.04.2021 22:00

Mathematics, 21.04.2021 22:00

Mathematics, 21.04.2021 22:00

Mathematics, 21.04.2021 22:00


SAT, 21.04.2021 22:00

English, 21.04.2021 22:00



Mathematics, 21.04.2021 22:00


Mathematics, 21.04.2021 22:00

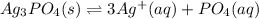
![Ksp=[Ag^+]^3[PO_4^-]](/tpl/images/0718/8186/a4d33.png)
 :
: