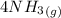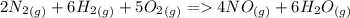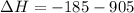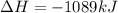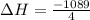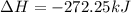Chemistry, 12.08.2020 06:01 Jazminfun70
Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) In the second step, ammonia and oxygen react to form nitric oxide and water: (g) (g) (g) (g) Calculate the net change in enthalpy for the formation of one mole of nitric oxide from nitrogen, hydrogen and oxygen from these reactions. Round your answer to the nearest .

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
List four observations that indicate that a chemical reaction may be taking place
Answers: 1

Chemistry, 22.06.2019 10:30
What is the empirical formula of c6h18o3? ch3o c2h5o c2h6o c2h5o5
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1
You know the right answer?
Nitric oxide (NO) can be formed from nitrogen, hydrogen and oxygen in two steps. In the first step,...
Questions


Mathematics, 24.05.2021 03:20

Biology, 24.05.2021 03:20


Mathematics, 24.05.2021 03:20

Law, 24.05.2021 03:20

Mathematics, 24.05.2021 03:20

Geography, 24.05.2021 03:20

Mathematics, 24.05.2021 03:20


Mathematics, 24.05.2021 03:20




Mathematics, 24.05.2021 03:20




Mathematics, 24.05.2021 03:20

Spanish, 24.05.2021 03:20

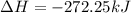 for 1 mole of NO.
for 1 mole of NO.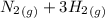 =>
=> 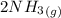
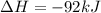
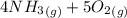 =>
=> 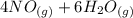

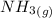 ) appeares as product in the first equation and as reagent in the 2 reaction, so when adding both, there is no need to inverse reactions. However, in the 2nd, there are 4 moles of that molecule, so to cancel it, you have to multiply by 2 the first chemical equation and enthalpy:
) appeares as product in the first equation and as reagent in the 2 reaction, so when adding both, there is no need to inverse reactions. However, in the 2nd, there are 4 moles of that molecule, so to cancel it, you have to multiply by 2 the first chemical equation and enthalpy: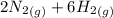 =>
=>