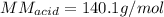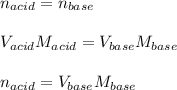Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Use examples from the article to explain one positive and one negative effect that chemistry has had on society.
Answers: 2

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1

Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3

You know the right answer?
A 0.753 g sample of a monoprotic acid is dissolved in water and titrated with 0.250 M NaOH. What is...
Questions

History, 20.09.2020 04:01


Chemistry, 20.09.2020 04:01

Physics, 20.09.2020 04:01




Mathematics, 20.09.2020 04:01



English, 20.09.2020 04:01


Mathematics, 20.09.2020 04:01





Mathematics, 20.09.2020 04:01