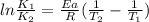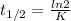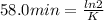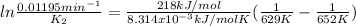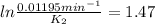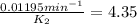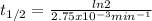Chemistry, 12.08.2020 05:01 alexmoy45p8yd7v
The decomposition of ethylene oxide(CH₂)₂O(g) → CH₄(g) + CO(g)is a first order reaction with a half-life of 58.0 min at 652 K. The activation energy of the reaction is 218 kJ/mol. Calculate the half-life at 629 K.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:10
Which of these will change if the air in aclosed bottle is heated? abcdthe mass of the airthe composition of the airthe air pressure in the bottlethe number of air molecules in the bottle
Answers: 3

Chemistry, 21.06.2019 21:30
An alcohol thermometer makes use of alcohol's changing in order to measure temperature. as the temperature goes up, the alcohol contained in the thermometer increases in volume, filling more of the thermometer's tube.
Answers: 3


Chemistry, 22.06.2019 14:30
How can carbon move from "land" to bodies of water? describe the way human impact has lead to increased levels of co2 in the atmosphere.
Answers: 2
You know the right answer?
The decomposition of ethylene oxide(CH₂)₂O(g) → CH₄(g) + CO(g)is a first order reaction with a half-...
Questions







Mathematics, 28.04.2021 23:50

Mathematics, 28.04.2021 23:50




Mathematics, 28.04.2021 23:50





Mathematics, 28.04.2021 23:50


Mathematics, 28.04.2021 23:50

Advanced Placement (AP), 28.04.2021 23:50