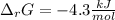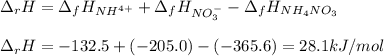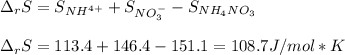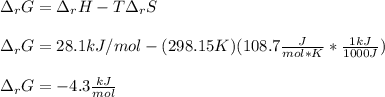Chemistry, 12.08.2020 05:01 apolloplays10
Consider the following chemical equation: NH4NO3(s)⟶NH+4(aq)+NO−3(aq) What is the standard change in free energy in kJmol at 298.15K? The heat of formation data are as follows: ΔH∘f, NH4NO3(s)=-365.6kJmolΔH∘f, NH+4(aq)=-132.5kJmolΔH∘f, NO−3(aq)=-205.0kJmol The standard entropy data are as follows: S∘NH4NO3(s)=151.1Jmol KS∘NH+4(aq)=113.4Jmol KS∘NO−3(aq)=146.4Jmol K Your answer should include two significant figures.

Answers: 3


Another question on Chemistry


Chemistry, 23.06.2019 02:30
Asubstance is held in an open container. its particles move past one another at random speeds but do not leave the container. heat is removed from the system, and the particles slow down. when enough heat is removed, the particles no longer have enough speed to overcome the weak attractive forces between them. when this happens, the substance enters its solid state. the process described above is known as .
Answers: 3

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 08:00
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
You know the right answer?
Consider the following chemical equation: NH4NO3(s)⟶NH+4(aq)+NO−3(aq) What is the standard change in...
Questions


Mathematics, 17.09.2019 08:30



Mathematics, 17.09.2019 08:30


Biology, 17.09.2019 08:30


Social Studies, 17.09.2019 08:30

Mathematics, 17.09.2019 08:30


English, 17.09.2019 08:30

Mathematics, 17.09.2019 08:30


English, 17.09.2019 08:30

Mathematics, 17.09.2019 08:30



History, 17.09.2019 08:30

Geography, 17.09.2019 08:30