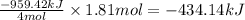Chemistry, 12.08.2020 04:01 21121212cutecheytown
4NH3(g) 5O2(g)4NO(g) 6H2O(g) Using standard thermodynamic data at 298K, calculate the free energy change when 1.81 moles of NH3(g) react at standard conditions.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 10:30
Astudent reacts 13 moles of iron with 21 moles of oxygen according to the following equation:
Answers: 1


Chemistry, 23.06.2019 12:50
Complete the paragraph to describe the characteristics of a borane molecule (bh3). the lewis structure and table of electronegativities are given. the bond polarities in bh3 are , the molecular shape is , and the molecule is .
Answers: 2
You know the right answer?
4NH3(g) 5O2(g)4NO(g) 6H2O(g) Using standard thermodynamic data at 298K, calculate the free energy ch...
Questions

Mathematics, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40

History, 16.12.2020 22:40


Biology, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40


Mathematics, 16.12.2020 22:40



Law, 16.12.2020 22:40


Health, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40

Mathematics, 16.12.2020 22:40



History, 16.12.2020 22:40