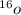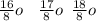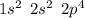. A certain diatomic element is a colorless gas and is highly reactive. It has three isotopes with mass #’s 16, 17 and 18 i. What is the naturally occurring diatomic element ? (2 points) ii. Write the isotopic symbols. (2 points) iii. How many neutrons does each possess? (2 points) iv. What is the electronic configuration of each isotope?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

Chemistry, 22.06.2019 17:30
A650 ml sodium bromine solution has a bromide ion concentration of 0.245 m. what is the mass (g) of sodium bromide in solution? a) 103.b)0.00155.c)16400.d) 16.4.e) 0.159
Answers: 2

Chemistry, 22.06.2019 23:00
What is the oxidation state of an individual bromine atom in nabro3?
Answers: 2

Chemistry, 23.06.2019 01:00
If a straight-chain hydrocarbon is a gas at room temperature, how many carbon atoms will it have? a. 6 carbon atoms b. 12 carbon atoms c. 24 carbon atoms d. 3 carbon atoms
Answers: 1
You know the right answer?
. A certain diatomic element is a colorless gas and is highly reactive. It has three isotopes with m...
Questions






Mathematics, 18.07.2020 23:01


English, 18.07.2020 23:01


Mathematics, 18.07.2020 23:01


History, 18.07.2020 23:01







Mathematics, 18.07.2020 23:01