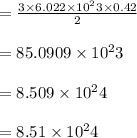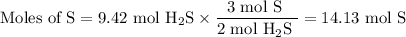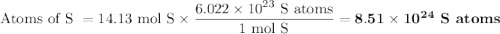Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:10
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 14:30
What type(s) of intermolecular forces are expected between ch3ch2cooh molecules? dipole forces, induced dipole forces, hydrogen bonding
Answers: 1

Chemistry, 22.06.2019 16:30
Ammonium perchlorate nh4clo4 is the solid rocket fuel used by the u.s. space shuttle. it reacts with itself to produce nitrogen gas n2 , chlorine gas cl2 , oxygen gas o2 , water h2o , and a great deal of energy. what mass of nitrogen gas is produced by the reaction of 2.1g of ammonium perchlorate?
Answers: 2
You know the right answer?
How many sulfur atoms are generated when 9.42 moles of H2S react according to the following equation...
Questions

World Languages, 10.10.2021 14:00

Mathematics, 10.10.2021 14:00


Chemistry, 10.10.2021 14:00

Mathematics, 10.10.2021 14:00

Law, 10.10.2021 14:00

English, 10.10.2021 14:00


Arts, 10.10.2021 14:00


Mathematics, 10.10.2021 14:00

Mathematics, 10.10.2021 14:00


Social Studies, 10.10.2021 14:00

Mathematics, 10.10.2021 14:00

English, 10.10.2021 14:00

Biology, 10.10.2021 14:00



Biology, 10.10.2021 14:00

 .
.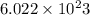 no of atoms & molecules. The calculation is as follows:
no of atoms & molecules. The calculation is as follows: