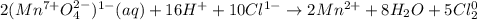Chemistry, 12.08.2020 08:01 Kaziyah461
After balancing the following reaction under acidic conditions, how many mole equivalents of water are required and on which side of the reaction do they appear?
MnO41- (aq) + Cl1- (aq) → Mn2+ (aq) + Cl2 (g)
a. 2 moles of H2O on the reactant side
b. 2 moles of H2O on the product side
c. 4 moles of H2O on the product side
d. 8 moles of H2O on the product side
e. 10 moles of H2O on the reactant side

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
If the particles in a sample of matter have an orderly arrangement and move only in place, the sample is a
Answers: 1

Chemistry, 22.06.2019 03:30
In saturated organic compounds, all the bonds between carbon atoms are called?
Answers: 1

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 2

You know the right answer?
After balancing the following reaction under acidic conditions, how many mole equivalents of water a...
Questions


Mathematics, 10.01.2021 18:40




Health, 10.01.2021 18:40

English, 10.01.2021 18:40

Mathematics, 10.01.2021 18:40



English, 10.01.2021 18:50

Social Studies, 10.01.2021 18:50

Mathematics, 10.01.2021 18:50


Biology, 10.01.2021 18:50

Mathematics, 10.01.2021 18:50




Physics, 10.01.2021 18:50

![MnO_4^{1-} (aq) + Cl^{1-} (aq) \rightarrow Mn^{2+} (aq) + Cl_2 (g)\\\\(Mn^{7+}O^{2-}_4)^{1-} (aq) + Cl^{1-} (aq) \rightarrow Mn^{2+} (aq) + Cl_2 (g)\\\\\\\\(Mn^{7+}O^{2-}_4)^{1-} (aq)+8H^++5e^- \rightarrow Mn^{2+}+4H_2O\\\\2Cl^{1-}\rightarrow Cl_2^0+2e^-\\\\2*[(Mn^{7+}O^{2-}_4)^{1-} (aq)+8H^++5e^- \rightarrow Mn^{2+}+4H_2O]\\\\5*[2Cl^{1-}\rightarrow Cl_2^0+2e^-]\\\\\\\\2(Mn^{7+}O^{2-}_4)^{1-} (aq)+16H^++10e^- \rightarrow 2Mn^{2+}+8H_2O\\\\10Cl^{1-}\rightarrow 5Cl_2^0+10e^-\\](/tpl/images/0720/0206/c85d6.png)