
Chemistry, 12.08.2020 08:01 Homepage10
Aqueous ammonia is added to a mixture of silver chloride and water. Given that Kf for the reaction between Ag+ and NH3 is large, which of the following are true?
A) The free ions are favored over the complex ion.
B) The complex ion is favored over solid silver chloride.
C) The free Ag+ ion is unstable.
D) More silver chloride will precipitate.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 13:10
What type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? view available hint(s) what type of interaction occurs between the r groups of valine and isoleucine in a tertiary structure? salt bridge disulfide bridge hydrogen bond hydrophobic interaction
Answers: 1

Chemistry, 22.06.2019 15:20
Identify arrows pointing to bonding electrons. done h-0-0-h ) intro
Answers: 3

Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
Aqueous ammonia is added to a mixture of silver chloride and water. Given that Kf for the reaction b...
Questions




Mathematics, 14.12.2020 21:00

Computers and Technology, 14.12.2020 21:00








Spanish, 14.12.2020 21:00


Health, 14.12.2020 21:00

Chemistry, 14.12.2020 21:00


Mathematics, 14.12.2020 21:00


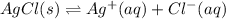
![Ag^+(aq)+2NH_3(aq)\rightleftharpoons [Ag(NH_3)_2]^+(aq)](/tpl/images/0720/0283/481b4.png)


