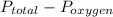Chemistry, 12.08.2020 18:01 Melymarrupe1345
Human lungs have evolved to breathe oxygen at a pressure as that in the atmosphere, 0.21 atm. If a particular heliox mixture to be carried by a scuba diver is at a pressure of 7.00 atm, what should be the partial pressure due to helium in order to maintain the pressure due to oxygen at 0.21 atm?

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1

Chemistry, 22.06.2019 17:30
Oil rich countries in the middle east cover about 4% of earths total land area but prossess about 48% of the worlds known oil reserves what is the main reason for high concentration of reserves in this part of the world
Answers: 3

Chemistry, 23.06.2019 06:30
What type of chemical reaction occurs between silver nitrate (agno3) and copper (cu)? the equation i was given is 2agno3 + cu —> 2ag+ cu(no3)2.
Answers: 1
You know the right answer?
Human lungs have evolved to breathe oxygen at a pressure as that in the atmosphere, 0.21 atm. If a p...
Questions


Business, 12.11.2019 17:31







Engineering, 12.11.2019 17:31

English, 12.11.2019 17:31



Social Studies, 12.11.2019 17:31

Social Studies, 12.11.2019 17:31

Chemistry, 12.11.2019 17:31

Mathematics, 12.11.2019 17:31


Mathematics, 12.11.2019 17:31


Chemistry, 12.11.2019 17:31

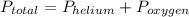 , where
, where 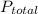 = total partial pressure of all the component gases in the mixture,
= total partial pressure of all the component gases in the mixture, 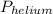 = partial pressure of helium gas, and
= partial pressure of helium gas, and 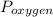 = partial pressure of oxygen gas.
= partial pressure of oxygen gas.