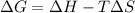Chemistry, 13.08.2020 04:01 janicemaxwell123
Any process with a negative change in enthalpy and a positive change in entropy will be:.
a. spontaneous
b. nonspontaneous
c. spontaneous at high temperatures
d. spontanteous at low temperatures

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 23:30
The density of the solid phase of a substance is 0.90 g/cm3 and the density of the liquid phase is 1.0 g/cm3. a large increase in pressure will a. lower the freezing point b. raise the freezing point c. lower the boiling point d. raise the triple point e. lower the triple point
Answers: 1

Chemistry, 23.06.2019 01:10
A5.00 g of a in . g of at aa 5.00 g of b in . g of .?at .
Answers: 1

Chemistry, 23.06.2019 04:00
What changes occur in the reaction indicated by the equation? check all that apply. the hydrogen nucleus loses protons. the oxygen nucleus gains protons. the bond in h2 is broken, and new bonds are formed between hydrogen and oxygen atoms. each electron associated with a hydrogen atom is shared with an oxygen atom.
Answers: 3

Chemistry, 23.06.2019 06:40
A250 g sample of water with an initial temperatureof 98.8 closes 6500 joules of heat. what is the finaltemperature of the water?
Answers: 1
You know the right answer?
Any process with a negative change in enthalpy and a positive change in entropy will be:.
a. sponta...
Questions



English, 18.05.2021 05:10

Mathematics, 18.05.2021 05:10

Mathematics, 18.05.2021 05:10



Mathematics, 18.05.2021 05:10





English, 18.05.2021 05:10


Mathematics, 18.05.2021 05:10


Mathematics, 18.05.2021 05:10

Geography, 18.05.2021 05:10

World Languages, 18.05.2021 05:10