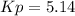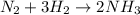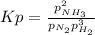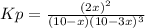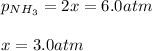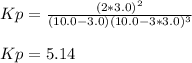Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 02:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3
You know the right answer?
a reaction mixture initially contains 10.0 atm N2 and 10.0 atm H2. If the equilibrium pressure of NH...
Questions

English, 15.01.2021 05:10

Mathematics, 15.01.2021 05:10

Mathematics, 15.01.2021 05:10

Mathematics, 15.01.2021 05:10

Mathematics, 15.01.2021 05:10


History, 15.01.2021 05:10


English, 15.01.2021 05:10






Mathematics, 15.01.2021 05:10

Mathematics, 15.01.2021 05:10

Mathematics, 15.01.2021 05:10

World Languages, 15.01.2021 05:10

Physics, 15.01.2021 05:10

Mathematics, 15.01.2021 05:10