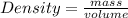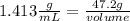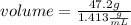Chemistry, 14.08.2020 01:01 reginaldboyd28
The density of concentrated nitric acid (HNO3) is 1.413 g/mL. What volume in liters would be occupied by a mass of 47.2 g?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2


Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 16:40
The diagram below shows the movement of particles. what does this piece of evidence best support? the collision theory the maxwell-boltzmann distribution the effect of pressure on reaction rates the effect of temperature on reaction rates
Answers: 3
You know the right answer?
The density of concentrated nitric acid (HNO3) is 1.413 g/mL. What volume in liters would be occupie...
Questions


Mathematics, 06.12.2021 05:20





Mathematics, 06.12.2021 05:20




Mathematics, 06.12.2021 05:20

Advanced Placement (AP), 06.12.2021 05:20


History, 06.12.2021 05:20


Mathematics, 06.12.2021 05:20

Mathematics, 06.12.2021 05:20


Biology, 06.12.2021 05:20

Mathematics, 06.12.2021 05:20