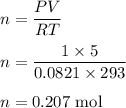Chemistry, 14.08.2020 01:01 rodriguezscarlet1713
PV = nRT. If P = 1 atm, V = 5.0 liter, R = 0.0821 L. atm/mol. K, and T = 293 K; what is the value of n?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
During chemistry class, carl performed several lab tests on two white solids. the results of three tests are seen in the data table. based on this data, carl has concluded that substance b must have bonds.
Answers: 2

Chemistry, 21.06.2019 22:30
Determine the wavelength of the light absorbed when an electron in a hydrogen atom makes a transition from an orbital in the n=3 level to an orbital in the n=7 level.
Answers: 2

Chemistry, 22.06.2019 04:00
The rules of engagement (roe) working group is often used to (select all that apply.)
Answers: 2

Chemistry, 22.06.2019 04:30
How many moles of air are there in a human lung with a volume of 2.4 l at stp? explain your answer
Answers: 1
You know the right answer?
PV = nRT. If P = 1 atm, V = 5.0 liter, R = 0.0821 L. atm/mol. K, and T = 293 K; what is the value of...
Questions


Mathematics, 29.03.2021 04:50

Mathematics, 29.03.2021 04:50


English, 29.03.2021 04:50

Biology, 29.03.2021 04:50

Social Studies, 29.03.2021 04:50


Physics, 29.03.2021 04:50


Mathematics, 29.03.2021 04:50


Social Studies, 29.03.2021 04:50

Biology, 29.03.2021 04:50

Spanish, 29.03.2021 04:50

Mathematics, 29.03.2021 04:50

Business, 29.03.2021 04:50

Mathematics, 29.03.2021 04:50


Mathematics, 29.03.2021 04:50