Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 19:00
Structure of the atoms: discovery of the nucleus in 1909i need answering all of these questions
Answers: 3

Chemistry, 23.06.2019 04:10
Two solids are mixed in a flask and stirred. after a few minutes, the flask becomes cold. which of the following best describes this reaction? a. an exothermic reaction b. a combustion reaction c. an endothermic reaction d. a decomposition reaction
Answers: 1
You know the right answer?
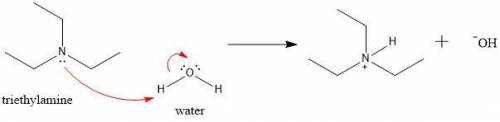
Write a net ionic equation to show that triethylamine, (C2H5)3N, behaves as a Bronsted-Lowry base in...
Questions


English, 11.11.2020 21:20


Mathematics, 11.11.2020 21:20

Physics, 11.11.2020 21:20



Mathematics, 11.11.2020 21:20

History, 11.11.2020 21:20

English, 11.11.2020 21:20

Mathematics, 11.11.2020 21:20






English, 11.11.2020 21:20



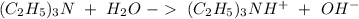
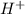 ).
). 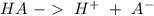
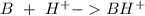
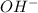 ).
).