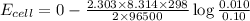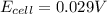Chemistry, 13.08.2020 18:01 scottcounts757
A concentration cell is one in which both the anode and cathode are the same but with different concentrations. Calculate the cell potential with [Zn2+] = 0.10 M[Zn2+] = 0.10 M for the cathode and the [Zn2+] = 0.010 M[Zn2+] = 0.010 M for the anode?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:10
Between 2014 and 2016, more than 25,000 children in flint, michigan, drank water that was contaminated with lead from lead pipes. during this time, the city claimed the water was safe to drink. which of these actions could the city have taken to ensure that the drinking water was free from lead?
Answers: 3

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1

Chemistry, 22.06.2019 18:00
Alidded glass container is filled with a colored gas. after a period of time, it is observed that the gas is uniformly spread throughout the box and that the movement has slowed considerably. next, a warm iron plate is carefully placed under the box. why is there resumed movement of the gas in the container?
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3
You know the right answer?
A concentration cell is one in which both the anode and cathode are the same but with different conc...
Questions


Mathematics, 02.07.2019 10:30

Biology, 02.07.2019 10:30

History, 02.07.2019 10:30

Biology, 02.07.2019 10:30

Mathematics, 02.07.2019 10:30


Mathematics, 02.07.2019 10:30


History, 02.07.2019 10:30




English, 02.07.2019 10:30

Business, 02.07.2019 10:30

Business, 02.07.2019 10:30


History, 02.07.2019 10:30

Arts, 02.07.2019 10:30

Computers and Technology, 02.07.2019 10:30

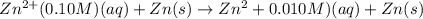
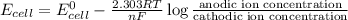
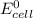 = standard electrode potential of the cell = 0 (as both metals are same )
= standard electrode potential of the cell = 0 (as both metals are same )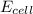 = emf of the cell = ?
= emf of the cell = ?