Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:30
For each of the following mixtures decide if filtering would be suitable to separate the substances. explain your answers. oil in water sugar in water sand in water chalk in water tea leaves in a cup of tea
Answers: 2

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 10:00
Drug abuse will not lead to physical and psychological dependence. true or false ?
Answers: 2
You know the right answer?
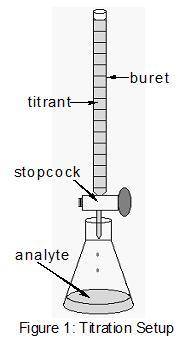
During a titration, the solution with the unknown concentration is called the ....
Questions


History, 25.06.2019 06:00


Mathematics, 25.06.2019 06:00

Mathematics, 25.06.2019 06:00


Mathematics, 25.06.2019 06:00


History, 25.06.2019 06:00

Mathematics, 25.06.2019 06:00

Mathematics, 25.06.2019 06:00

Mathematics, 25.06.2019 06:00


English, 25.06.2019 06:00



Biology, 25.06.2019 06:00

Computers and Technology, 25.06.2019 06:00


Mathematics, 25.06.2019 06:00