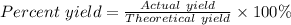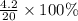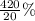Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:30
Which common material is an example of a polymer? (25 pts) a. steel b. plastic c. petroleum d. rubbing alcohol
Answers: 2

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 23.06.2019 00:30
Titration reveals that 11.6 ml of 3.0m sulfuric acid are required to neutralize the sodium hydroxide in 25.00ml of naoh solution. what is the molarity of the naoh solution?
Answers: 1

Chemistry, 23.06.2019 06:00
What are the coefficients to balance the following equation? ba+br2=babr2
Answers: 2
You know the right answer?
If 10.0 moles of O₂ are reacted with excess NO in the reaction below, and only 4.2 mol of NO₂ were c...
Questions

Mathematics, 01.05.2021 01:00




Computers and Technology, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00





Mathematics, 01.05.2021 01:00







Mathematics, 01.05.2021 01:00

Mathematics, 01.05.2021 01:00