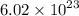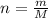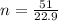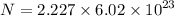Chemistry, 15.08.2020 21:01 Ellafrederick
Determine the number of atoms in 51.0 grams of sodium, Na. (The mass of one mole of sodium is 22.99 g.)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:50
Problem page gaseous ethane reacts with gaseous oxygen gas to produce gaseous carbon dioxide and gaseous water . if of water is produced from the reaction of of ethane and of oxygen gas, calculate the percent yield of water. be sure your answer has the correct number of significant digits in it.
Answers: 2

Chemistry, 21.06.2019 22:20
Calcium hydride (cah2) reacts with water to form hydrogen gas: cah2(s) + 2h2o(l) → ca(oh)2(aq) + 2h2(g) how many grams of cah2 are needed to generate 45.0 l of h2 gas at a pressure of 0.995 atm and a temperature of 32 °c?
Answers: 2

Chemistry, 22.06.2019 07:40
The formation of a solid, also known as a is an indication of a chemical change. precipitate particulate particle powder
Answers: 3

Chemistry, 22.06.2019 09:40
How many grams of aluminum will there be in 98g of al2o3?
Answers: 1
You know the right answer?
Determine the number of atoms in 51.0 grams of sodium, Na. (The mass of one mole of sodium is 22.99...
Questions

Physics, 23.10.2020 14:00

Mathematics, 23.10.2020 14:00



Mathematics, 23.10.2020 14:00



Mathematics, 23.10.2020 14:00



Social Studies, 23.10.2020 14:00


Mathematics, 23.10.2020 14:00



Mathematics, 23.10.2020 14:00



Mathematics, 23.10.2020 14:00

English, 23.10.2020 14:00