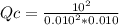Chemistry, 16.08.2020 01:01 longoriafaithe09
At 700 K, the reaction 2SO 2(g) + O 2(g) 2SO 3(g) has the equilibrium constant K c = 4.3 × 10 6, and the following concentrations are present: [SO 2] = 0.010 M; [SO 3] = 10. M; [O 2] = 0.010 M. Which of the following is true based on the above?
A. Qc < Kc, the reaction proceeds from right to left to reach equilibrium
B. Qc < Kc, the reaction proceeds from left to right to reach equilibrium
C. Qc > Kc, the reaction proceeds from right to left to reach equilibrium
D. Qc > Kc, the reaction proceeds from left to right to reach equilibrium
E. Qc = Kc, the reaction is currently at equilibriums

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 10:30
What woukd most likely be the transmittance at a 0.70 m solution of solute a? a) 7.6%b) 1.1%c)4.0%d)4.6%
Answers: 1

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
At 700 K, the reaction 2SO 2(g) + O 2(g) 2SO 3(g) has the equilibrium constant K c = 4.3 × 10 6, and...
Questions



Mathematics, 07.12.2019 07:31


History, 07.12.2019 07:31

Social Studies, 07.12.2019 07:31




Mathematics, 07.12.2019 07:31




History, 07.12.2019 07:31

Biology, 07.12.2019 07:31


Mathematics, 07.12.2019 07:31



History, 07.12.2019 07:31

![Qc=\frac{[C]^{c}*[D]^{d} }{[A]^{a} *[B]^{b} }](/tpl/images/0723/0556/1eb27.png)
![Qc=\frac{[SO_{3} ]^{2} }{[SO_{2} ]^{2} *[O_{2} ]}](/tpl/images/0723/0556/691d7.png)